Lu-177 PSMA
What is Lu-177 PSMA Therapy?
An emerging targeted treatment for patients with advanced prostate cancer. it is currently used when the disease has metastasised and when other treatment options are poorly tolerated or have failed
How does Lu-177 PSMA work?
A form of radionuclide treatment that targets prostate specific membrane antigen (PSMA). PSMA is a cell surface membrane protein which is overexpressed in 90 to 95% of prostate cancer cells whilst not expressed in most of the normal tissues. By targeting PSMA with radioactive nuclides, PSMA expressing prostate cancer cells can be selectively targeted and neutralized whilst minimizing the effects on normal tissue
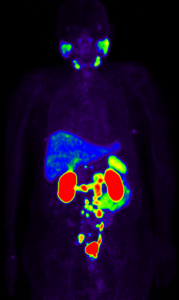
PSMA PET/CT
Pretreatment PSMA PET/CT shows intensely PSMA-avid abdominal and pelvic lymph nodes
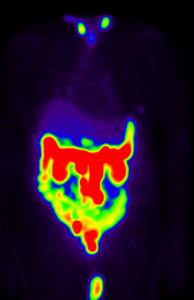
1st Cycle Lu-177 PSMA
SPECT-CT performed 24 hours post 177Lu-PSMA treatment shows good tracer localization within the PSMA-avid abdominal and pelvic lymph nodes

2nd Cycle Lu-177 PSMA
SPECT-CT performed 24 hours post 177Lu-PSMA treatment of 2nd cycle of 177Lu-PSMA shows good tracer localization within the residual abdominal and pelvic lymph nodes. These lymph nodes are similar and less PSMA-avid in keeping with a good treatment response.
Is this treatment safe?
As most of the normal tissues do not express PSMA, the side effects are minimized. During treatment, some patients may experience nausea or vomiting. Patients may also experience fatigue and flare of symptoms (e.g. bone pain) typically within a week following treatment. Following a few cycles of treatment, some patients may experience some mouth dryness due to presence of PSMA expression in the salivary glands. Patients with widespread bone disease may also experience a reduction in blood counts following treatment.
When should I consider this Therapy?
Currently, this treatment is offered to patients with metastatic prostate cancer that is not responsive to conventional treatment, patients who are not fit to undergo hormonal treatment or chemotherapy due to concurrent cardiac or kidney illnesses or patients who are unable to tolerate the side effects of conventional therapy. Patients who are interested to find out more about the treatment can call us at our hotline 6708 7888 to make an appointment to see our doctor.
RADIUM 223 (XOFIGO)
What is Radium-223 Dichloride (Xofigo®) Therapy?
Radium-223 therapy is a form of radionuclide therapy used mainly in patients who have castrate resistant prostate cancer that has spread to the bones. The main aim of this treatment is to relieve bone pain caused by the cancer. Studies have shown that there is an added overall survival benefit compared to best supportive care.
How Does Radium-223 Therapy Work?
Radium-223 is a radioactive particle which releases a type of radiation called alpha radiation. Radium-223 shares similar chemical properties to calcium and targets areas of high bone turnover which is seen as a response in bone to most cancer types. Upon administration, Radium-223 travels to the areas of high bone turnover and emits alpha radiation unto the surrounding cancer cells.

Image source: https://www.xofigo-us.com/patient/how-xofigo-works/
How Xofigo® Is Given:
- Xofigo is given through a vein (intravenously, IV), as a slow [intravenous] injection, over 1 minute.
- In a clinic or facility with trained healthcare providers.
- It is given once every 4 weeks, total of 6 doses.

Is This Treatment Safe?
Alpha particles penetrate very short distances (two to ten cells in depth), reducing bystander damage to adjacent healthy tissue. Main side effects would include a temporary reduction in the number of blood cells produced and infrequently, symptoms of nausea, vomiting and diarrhoea.
When Would I Consider Using this Treatment?
This treatment has been approved by the FDA (US) for used in patients with metastatic castrate resistant prostate cancer with bone metastases. It’s used in bone metastasis from other cancer types eg breast, bone forming sarcomas, are currently still under investigation. Please check with your doctor regarding suitability for treatment.